Abstract
Patients with Chronic Granulomatous Disease (CGD) have an inherited defect in production of microbicidal oxidants by neutrophils and monocytes that can result in life-threatening infections. Some of these infections, particularly those with mold fungi that invade across tissue boundaries, may persist and progress despite intensive medical therapy. In such a setting there is a long history of published single case and small series reports suggesting that a course of unmatched allogeneic donor granulocyte (gran) transfusions may result in improvement or even resolution of such refractory life-threatening infections in CGD patients. Donors for grans transfusion products are often challenging to find and not HLA-matched. Because of this most CGD patients can be provided only a limited course of grans transfusions before they develop limiting anti-HLA antibodies or lack of available donors.
To address this problem, we developed an ex vivo correction of NADPH oxidase defective grans from CGD by mRNA transfection1. Apheresis grans transfected with mRNA restored NADPH oxidase function in vitro and following transplant into immunodeficient mice. Messenger RNA-transfected autologous grans from non-human primates (NHP) were well tolerated and were detected for up to 5 days in NHP peripheral blood following infusion. This pre-clinical work was transitioned to a Phase I, dose-escalating, feasibility and safety study (NIH Protocol 22-I-0001; ClinicalTrials.gov NCT05189925). We report here data from the first X-linked CGD subject infused with autologous gp91 mRNA-transfected grans at the first dose level. The patient received two daily doses of G-CSF before apheresis. From 26 x10e9 total nucleated cells in the apheresis product, ~7x10e9 grans were collected, though only 1.5x10e9 grans were subjected to electroporation transfection with gp91phox mRNA (CELLSCRIPT LLC). A single infusion of 70e6 (10e6 per kg) mRNA-transfected autologous grans where about 57% were DHR+ (40x10e6 DHR positive grans) was well tolerated without any adverse effects. NADPH oxidase functional (DHR+) grans were detected in peripheral blood (PB) at last assessment (72 hrs) after this single infusion of mRNA corrected autologous grans.
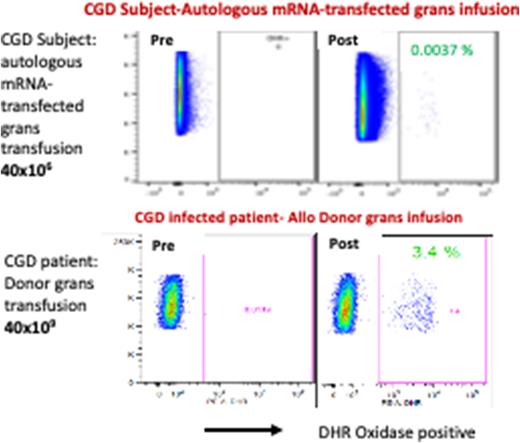
Serial PB evaluation post infusion from study Subject detected oxidase (DHR)+ grans. Of note, DHR+ grans in Subject PB at ~ 43 hrs after infusion was 0.0037% compared to his pre-infusion DHR assays (0%) (Figure, top row). As a comparator, DHR assays performed pre- and also ~ 40 hrs post infusion of a clinical product of 40x10e9 allogeneic donor grans into another CGD patient being treated clinically for a refractory infection is shown (bottom row). There is 1000 fold more allogeneic DHR+ grans infused for the clinical treatment than the experimental DHR+ mRNA corrected autologous grans infused. Compared to their corresponding absolute neutrophil count, the study Subject (received 40x10e6 DHR+ mRNA transfected autologous grans) had 512 DHR+ neutrophils/ml while the CGD patient (received clinical 40x10e9 allogeneic grans) had 79,560 DHR+ neutrophils/ml. This indicates there are > 6 fold more mRNA-corrected autologous grans in circulation at 43 hrs per cell infused than following the clinical treatment with allogeneic cells infused. Our data demonstrates that mRNA-corrected autologous grans are not only well tolerated but are very well sustained viable and functional in the circulation relative to clinical unmanipulated allogeneic donor grans. Additional patients need to be studied at this dose level and the escalating dose levels to assure that this could be a safe alternative to non-HLA matched allogeneic grans transfusions.
Figure shows FACS DHR analysis of peripheral blood from CGD patients after infusion of autologous mRNA-transfected granulocytes 40x106 (top) and donor granulocytes 40x109 (bottom).
Disclosures
Meis:CELLSCRIPT LLC: Current Employment. Dahl:CELLSCRIPT: Current Employment, Current equity holder in private company. Malech:Prime Medicine: Research Funding; CSL Behring: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal